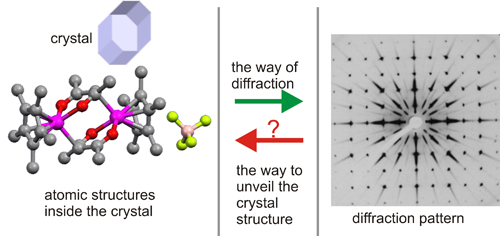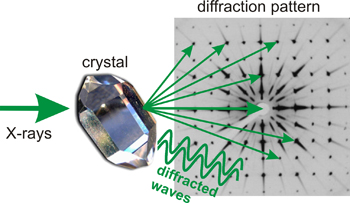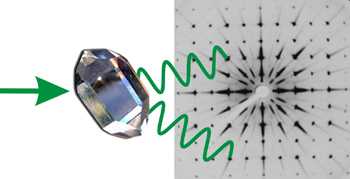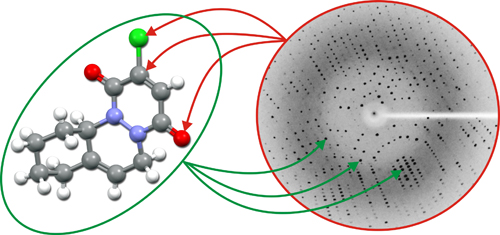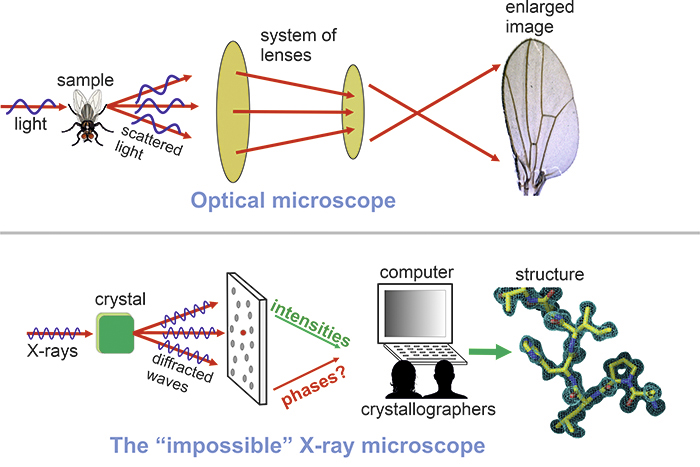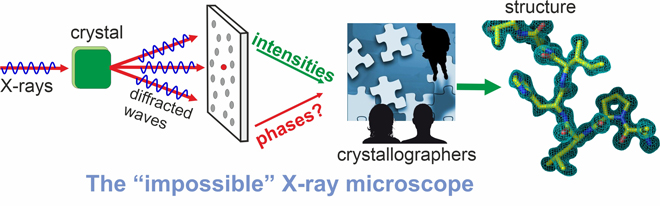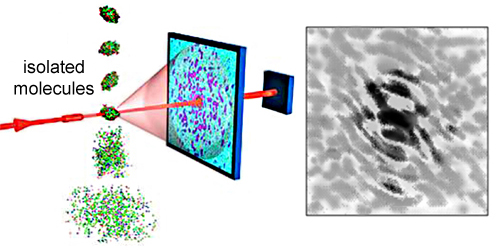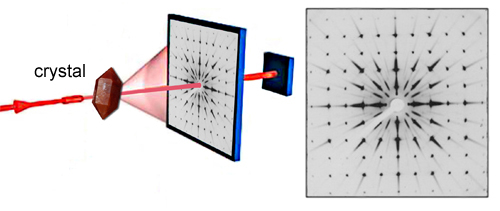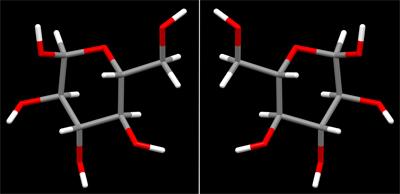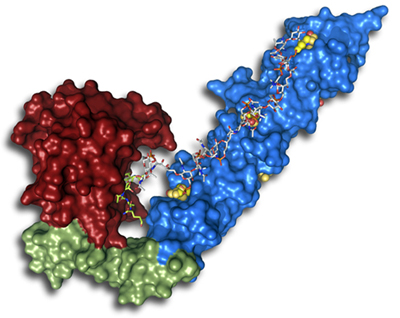
If
you don't have time or you are not keen to start reading all the
information offered through the menu on the left (or in the table of
contents), here you will find
answers for a few questions that will help you to
understand the beauty and capabilities of this part of science
known as Crystallography: